Pipeline
NEUROBIOGEN has been working with an advisory network of researchers and clinical
experts specializing in brain & neurological diseases with long experience.
experts specializing in brain & neurological diseases with long experience.
Pipeline Overview
Pipeline Overview
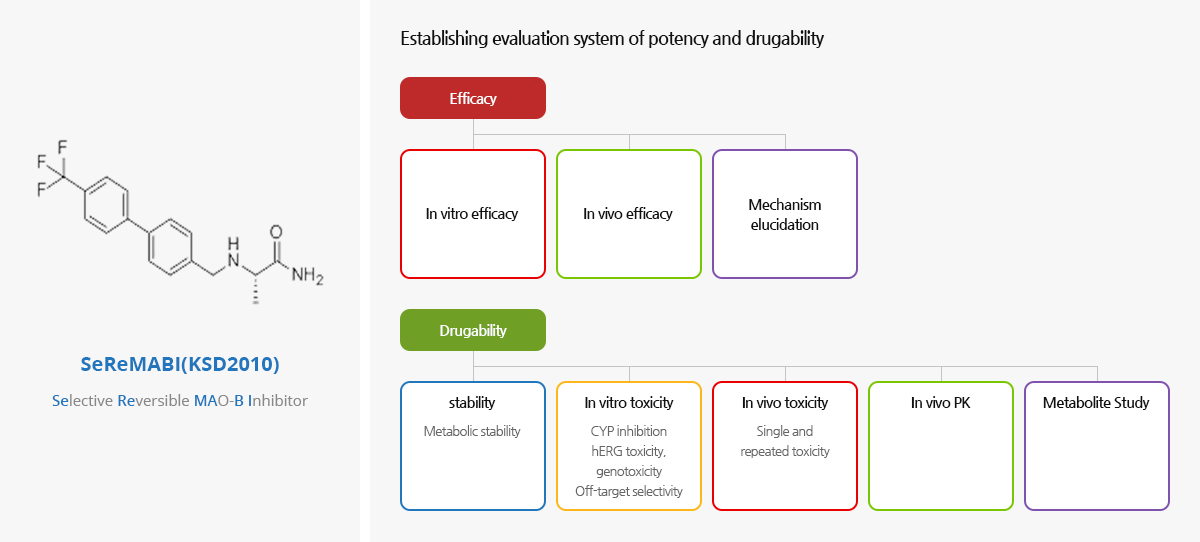
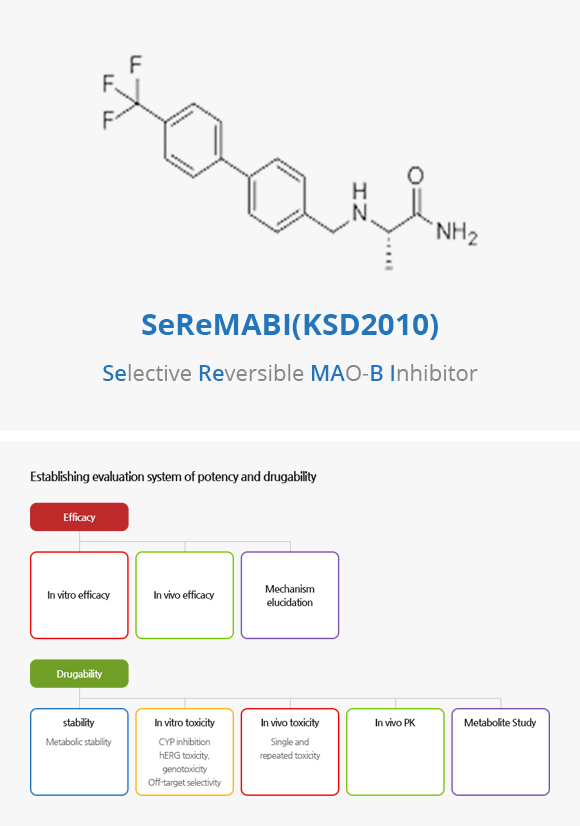
SeReMABI(KDS2010) Project
NEUROBIOGEN's newly-developed material (SeReMABI, Selective Reversible MAO-B Inhibitor/KDS2010) reversibly and selectively blocks the abnormal synthesis of inhibitory neurotransmitter (GABA) in reactive astrocytes, and this innovative new drug targets the cure of brain diseases and central nervous system diseases.
Based on this effect mechanism, we have confirmed the strong potency of SeReMABI by non-clinical trials as a treatment for a rare disease such as spinal cord injury including stroke sequelae, and Parkinson's.
Based on this effect mechanism, we have confirmed the strong potency of SeReMABI by non-clinical trials as a treatment for a rare disease such as spinal cord injury including stroke sequelae, and Parkinson's.
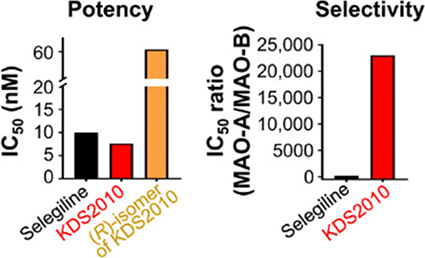
Potent & Selective Inhibitor, SeReMABI is the best-in-class MAO-B inhibitor.
| Compound | hMAO-B (IC50) | hMAO-A (IC50) | Selectivity (MAO-A/MAO-B) | |
|---|---|---|---|---|
| SeReMABI | 7.6 nM | > 100 μM | > 10000 | Reversible, very selective |
| Safinamide | 79 nM | > 80 μM | > 1013 | Reversible, but multi-target |
| RG-1577 | 15 nM(6 nM*) | 3.85* μM | ~ 653 | Reversible, not selective |
| Selegiline | 10 nM(9 nM*) | 1.5 μM* | ~ 150 | Irreversible, not selective |
| rasagiline | 14 nM* | 710 nM* | ~ 50 | Irreversible, not selective |
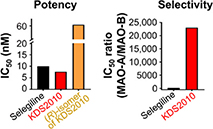
Potency and selectivity of selegiline and SeReMABI based on IC50 (in nM) levels of MAO-B and the isoform MAO-A.
| Efficacy test | Patent (domestic) | Patent (abroad) | Publish | ||
|---|---|---|---|---|---|
in progress |
01Alzheimer's disease | Complete | Registered | Registered in USA, Canada, Australia, Japan, Russia, 1)EU, China | Nature Medicine(2014.06) Science Advance(2019.03) |
| 02Post stroke | Complete | Registered | Registered in USA, Canada, Australia, Japan, Russia, 1)EU, China | Cell Reports(2020.07) | |
| 03Spinal cord injury | Complete | Registered | Registered in USA, Canada, Australia, Japan, Russia, 1)EU, China | Cell in progress | |
| 04Obesity | Complete | Registered | Registered in USA, Canada, Australia, Japan, Russia, 1)EU, China | ||
| 05Traumatic brain injury | Complete | Patent pending | |||
| 06Parkinson's disease | Complete | Registered | Registered in USA, Canada, Australia, Japan, Russia, 1)EU, China | Molecular Therapeutics in progress |
|
| 1) EU (8 countries): France, UK, Germany, Switzerland, Spain, Italy, Portugal, Belgium | |||||
Expected |
07Multiple sclerosis | in progress | |||
| 08Amyotrophic lateral sclerosis | in progress | ||||

